Introduction
Importance of recombinant proteins have been increased 100 creases. Because of the promotions in rDNA engineering, they are found in every pharmaceutics, physician ‘s clinic, medical and biological research research lab.
In this reappraisal we have discussed the processs involved in doing this recombinant protein. Get downing from a cistron sequence and stoping up in doing purified protein in bulk measures. The stairss included in while recombinant Protein production includes the 1 ) cistron sequence of coveted protein, 2 ) vector system choice & A ; readying 3 ) film editing of vector & A ; aim cistron by limitation enzymes & A ; ligation to organize recombinant DNA, 4 ) Transformation into host E coli ( 5 ) Selection & A ; showing of transformed Ecoli ( 6 ) purification of proteins. Downstream processing of proteins is besides really critical & A ; challenging, because for crystallography, NMR & A ; Mass spectroscopy extremely purified proteins are required. Proteins have different belongingss which are used to do the purification procedure easier, separation of proteins can be made based on their charge, Size & A ; pH. Methodology & A ; rules of different chromatographic techniques including, size exclusion chromatography, ion exchange column chromatography, high public presentation liquid chromatography, reversed-phase chromatography were discussed every bit good. Furthermore, the hereafter applications of protein molecular biological science and its importance in medical specialty & A ; industry were besides discussed.
Molecular cloning is the type of cloning, which involves the reproduction of one molecule to bring forth a population of cells with a big figure of indistinguishable DNA molecules ( Oldenberg J & A ; Dolan G 2000 ) .
Principle of Molecular Cloning: The Principle of molecular Cloning involves the transmutation of R DNA in host cell e.g E coli. When the host cell divides the Deoxyribonucleic acid molecule undergoes reproduction & A ; goes with the host cell. ( Am J hosp 2000 ) .
Methods of Molecular Cloning
- In vivo, in which the usage of Enzymes like limitation enzymes and ligases, and vectors is required
- The other type is in vitro, in which, the polymerase concatenation reaction ( PCR ) method is used to make transcripts of fragments of DNA.
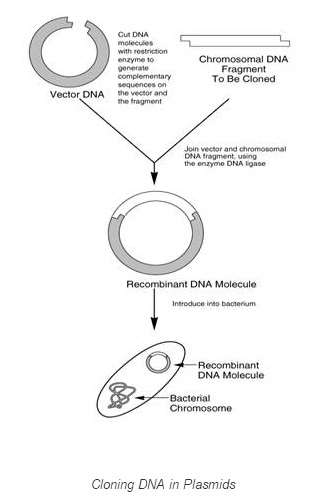
Figure 1. Genome Management Information System, Oak Ridge National Laboratory, U.S. Department of Energy Genome Programs hypertext transfer protocol: //genomics.energy.gov
Application of molecular cloning
- To bring forth utileproteins for curative intents
- Defective cistron therapy e.g. in instance of heamophilia, factor VIII is faulty and adequate insulin is non produced in instance of diabetes ( Lewandowski C & A ; Barsan W 2001 ) .
- For intervention of dangerous diseases e.g. to handle shots, tissue plasminogen activator is used
- For the production of Subunit vaccines- to immunise patients without exposing them to whole being ( e.g. hepatitis B vaccinum.
- Whole genome sequencinghas been possible because of molecular cloning technique. Because of this, the complete DNA sequence of an being ‘s genome can be found at a individual clip. Mitochondrial & A ; chloroplast Deoxyribonucleic acid. Have been sequences every bit good.
- Peptide sequencing, or amino acerb sequence, is the technique in which amino acid sequence of a protein can be found. Both terminals, N-terminal & A ; C-terminal, can be determined by peptide sequencing. The N-terminal terminal has a free amino group and the C-terminal terminal contains free carboxyl group..
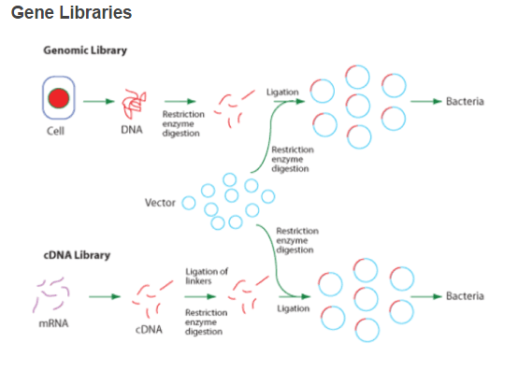
- Acomplementary DNA libraryrepresents the entire cistrons expressed in the cell population. RT is used to bring forth messenger RNA. The ensuing complementary DNA can be futher cloned in a suited host like E.coli ( as seen in the diagram below ) . As the complementary DNA is made after RNA treating so it merely includes the sequences that encode the protein.
- Chromosome walkingis a type of cloning method in which peculiar allelomorph of any cistron can be located & amp ; cloned. It is besides termed as positional cloning. It can research the desire protein in an chartless sequence e.g. a peculiar allelomorph involved in familial disease can be found by chromosomal walking. The rule of this technique is that those cistrons who are already identified, walking starts from that cistron. That cistron is besides known as a marker cistron ( Bartlett & A ; J. M. 2003 ) .
Manipulation of recombinant Deoxyribonucleic acid
Recombinant DNA ( rDNA ) is a Deoxyribonucleic acid molecule incorporating Deoxyribonucleic acid from two different species.Principle:
Deoxyribonucleic acid of two different species can be ligated together for utile intents. DNA molecule & A ; vector are treated with Restriction enzyme digestion & A ; are joined together via familial recombination to do rDNA ( : a B Saiki, R. & A ; Scharf 2013 ) .
Stairss in theDevelopment of Recombinant DNA
In order to organize an rDNA, following things are required
- Deoxyribonucleic acid of involvement
The Deoxyribonucleic acid of protein of involvement can be extracted from the coveted being genome. The Deoxyribonucleic acid is so purified in order to take polluting proteins, RNA ( ribonucleinase ) and smaller molecules. Polymerase concatenation reaction ( PCR ) can besides be used for elaboration of specific DNA or RNA.
- PCR
Polymerase Chain reaction was developed by Kary Mullis in the 1980s ( Saiki, R & A ; Gelfand 2002 ) .The chief involved in PCR is to synthesise new strand of DNA complementary to the templet strand, by utilizing the ability of DNA polymerase. Billions of transcripts can be produced in this manner.
PCR has a assortment ofapplications in medical and biological research( Freshney, Ian R 2004 ) Including DNA cloning, functional analysis of genome, Identification of familial upsets etc.
Cloning vector
A cloning Vector is a Deoxyribonucleic acid molecule that contain necessary familial signals for reproduction, sequences for infixing foreign Deoxyribonucleic acid and has the ability to show the foreign DNA ( lambrook, J. and D. Russell. 2001 ) .
Vectors are by and large derived from plasmids or viruses.
Plasmid Vectors: Plasmids are double-stranded, self-replicating, round Deoxyribonucleic acid molecules
Plasmid vectors consist of an
- Beginning of reproduction,
- Multiple cloning sites & A ; a selectable marker cistron
Plasmids can undergo DNA transportation by either through junction or They may non make it by junction therefore called as non-conjugative.e.g. , many R and col plasmids.
Viral vectors
Noninfectious viruses transporting modified viral Deoxyribonucleic acid or RNA are besides used as vectors. It contains viral boosters and besides the transgene, which can be translated under booster. Some of the vectors for illustration retroviruses, are used for lasting integrating into the host genome by their characteristic retroviral form of integrating ( Campbell, Neil A. & A ; Reece, Jane B.. 2002 ) .
Besides retroviruses many different viruses are used as vectors like adenoviruses, adeno associated viruses etc.
- Restriction enzymes/ligase
Restriction endonucleases are protein produced by bacteriums that has the ability to split DNA at specific sites. Restriction enzymes can be isolated from bacterial cells and used in the research lab to pull strings fragments of DNA, for illustration to insulate cistron fragment from any genome & A ; besides it is used extensively in the production of rDNA engineering. ( Peter Walter 2008 ) .
Protein look in E. coli
Thecistrons or Deoxyribonucleic acid to be cloned is inserted into the vector at multiple cloning sites ( MCS ) . The limitation sites in the MCS of vector are cleaved by the same limitation enzymes as of mark cistron. Dna ligase is used to fall in their cutted terminals.DNA is so injected into E.coli via assorted methods, including transmutation, transduction, transfection or electroporation ( Brown, Terry ( 2006 ) ( Russell, david 2001 ) .
The efficiency of transmutation does non depend on how the recombinant Deoxyribonucleic acid is introduced into the host. The uptake frequence of cells is normally low. So a really little proportion of cells undergo transmutation.
Selectable marker: In order tochoose the positively transformed cells choice marker is used. Antibiotic opposition is frequently used as a selectable marker ; an illustration is the beta-lactamase cistron which confers opposition against Principen. Some vectors contain two selectable markers, for illustration the plasmid pACYC177 has both Principen and kanamycin opposition cistron ( Nicola & A ; Andrew 2005 ) .
Merely those cells that contain the selectable marker cistron, will last because it confers opposition when exposed to the antibiotic, while those that have failed to take up plasmid sequences will decease.
Reporter genes/Fusion Proteinsare besides used in some cloning vectors in order to ease the showing of transformed ringers by utilizing characteristics of these cistrons that allow successful ringer to be easy identified. Green fluorescent protein ( GFP ) and luciferase cistrons are used as newsman cistrons ( Philippe Bernard 2006 ) .
Protein Expression
Blue-white showing system is the most common method, used to separate between the cells that has the desired vector ( holding Gene of involvement ) and those transformed cells in which vector is present but the coveted cistron is non in the vector molecule. For this screening cistron sequences is inserted in cistron encryption of beta-galactosidase enzyme, who really gives bluish coloured merchandise in civilization medium. Interpolation of the foreign DNA into the beta-galactosidase cryptography sequence disables the map of the enzyme, so that settlements incorporating transformed DNA go white. In this manner, transgenic bacterial ringers are easy identified ( Esposito D, Garvey LA 2009 ) .
Protein look can be controlled in bacterium by the Lac Operon. Bacteria, when grown in lactose, start metabolising it and do the production of two different proteins, permease & A ; Beta-galactosidase ( Gabant P & A ; Van Reeth 2000 ) . Protein of involvement can be cloned in this part, under lac operon by utilizing IPTG as protein inducer.
Protein purification
In order to obtain a protein with coveted structural & A ; functional belongingss, it must be at least 98 % purified. There are many different techniques involved for the separation of different proteins.Separation stairss normally exploit differences in protein size, physico-chemical belongingss, adhering affinity and biological activity.
Separation based on isoelectric point
Principle:
Proteins can be separated based on the nature and grade of their ionic charge.
In Ion exchange chromatographythe column is selected harmonizing to the type and strength of charge. Positively charged rosins are used to divide negatively charged proteins, while negative charged rosins are used to divide positively charged compounds ( Kennedy, RM 2008 ) .
Methodology: Buffer is pumped in the column to neutralize the opposing charged ions. When the sample is injected in column, solute molecules exchange with the buffer ions and competes for the binding sites on the rosin. Retention clip for each solute depends upon the strength of its charge. Weakly charged compounds will elute foremost, followed by those who have stronger charges.
ApplicationsProtein purification
Separation based on Size
Size exclusion Chromatographyutilizations porous beads in the signifier of gels to divide protein in solution or denaturing conditions ( Ehle H horrn A 2003 ) .
Principle: proteins of big size will easy go throughthrough the column and emerge/elute foremost.
Methodology: Smaller molecules take a longer clip because they will acquire entrapped in gel beads. In the context of protein purification, the eluent is normally pooled in different columns and those holding small hints of the protein are discarded.
Application
Fractionation of proteins and other H2O soluble polymers
Separation by mutual opposition
Principle:base on the belongings of specific chemical groups for high affinity
Methodology: In Affinity Chromatographyspecific rosins are used for separation.These rosins have ligands attached to their surfaces which are specific for the compounds to be separated. For illustration, membrane proteins can be purified by lectin affinity chromatography.
Application
Production of pure antibodies
High public presentation liquid chromatography as the name indicates itis a type of chromatographic technique that uses high force per unit area to drive the solutes through the column faster. By this diffusion of atoms becomes limited and the declaration is improved. The most common signifier is “ reversed stage ” HPLC, in which hydrophobic column is applied.The proteins undergo elution by pouring increasing concentrations of an organic dissolver. The proteins are eluted based on their hydrophobic charges. ( Regnier FE 1983 ) .
Applications:it has many applications in nutrient & A ; pharmaceutical industry.
Importance of pureness for crystallography, NMR and mass spectroscopy
For the structural designation of protein crystals by NMR & A ; mass designation by mass spectroscopy, extremely purified proteins are required.Proteins must be 98 % pure. Purity can be checked by running the protein mixtureon SDS-PAGE prior to crystallisation. If the protein is non purified, than the folding form by NMR and besides the construction of protein crystal will acquire affected ( Christopher G. Tate 2009 ) .
Drumhead
Proteins are the most abundant and diverse molecules found in life cells. Due to promotions in protein molecular biological science techniques, we are able to bring forth proteins with new or desirable maps via assorted protein technology techniques. Almost all enzymes are protein in nature, involved in commanding assorted activities inside the cell/body. So over the period of old ages assorted promotions have been made in this field.
In 1983, ulmer, the combination of crystal construction and protein chemical science information with unreal cistron synthesis was emphasized as a powerful attack to obtain proteins with desirable Properties. And now due to the development in recombinant DNA engineering and high-throughput showing techniques, protein technology methods and applications are going progressively of import and widespread. . In medical specialty, familial technology has been used for the extended production of recombinant insulin for diabetes, for bring forthing different growing endocrines, endocrines for the intervention of sterility, for the production of of import human proteins, different vaccinums and many other drugs ( John C. Avise ( 2004 ) .enormously larger measures of recombinant proteins are produced in big bioreactors in which transformed being are grown by utilizing different techniques of industrial agitation, and so by downstream processing they can be purified.





