| Chapter 1.0 |
| Introduction |
- Tetraspanins
Tetraspanins are the little cell surface proteins ( Hemler, 2005 ) that can be found over a great extent in all metazoans ( Huang et al. 2005 ) . Until today, scientists have discovered 33 members of tetraspanin superfamily expressed in worlds and mice ( Charrin et al. 2009 ) . Tetraspanins are expressed in all human cell types and can be found in the exocytotic tract, cell surface, endosomal system, secretory lysosomes ( Pols and Klumperman, 2009 ) and they are enriched in exosomes ( van Niel et Al. 2006 ) . The tetraspanin protein construction comprises of four transmembrane spheres attached to a short extracellular cringle ( EC1 ) , a really short intracellular cringle, and a longer extracellular cringle ( EC2 ) ( Hemler, 2005 ) (Figure 1.1 ). This protein construction is flanked by relatively short N- and C-terminal cytoplasmic dress suits ( Hemler, 2005 ) . Except the 2nd transmembrane sphere, the other transmembrane spheres of the most tetraspanins undergo palmitoylation to their membrane-proximal cysteine residue during post-translational alteration ( Hemler, 2005 ) . The add-on of palmitate enables the tetraspanins to tie in with each other and assorted built-in proteins, forming an interacting web at the cell surface called “tetraspanin-enriched microdomains” ( TEMs ) ( Rubinstein et al. 1996 ; Hemler, 2005 ; Charrin et Al. 2009 ) . Within TEMs, besides known as “tetraspanin web” , tetraspanins act as molecular facilitator, interacting with integrins and other proteins to enable them interceding the cells in extracellular matrix ( ECM ) for adhesion, cell motility, invasion, merger and signalling ( Boucheix and Rubinstein, 2001 ; Hemler, 2003 ) .
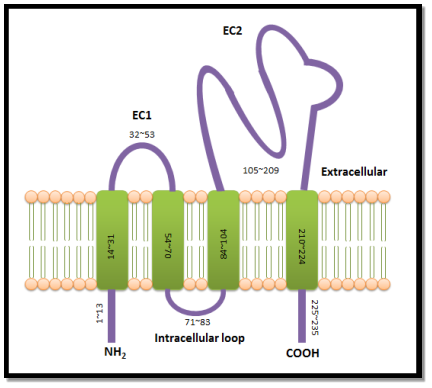 |
| Figure 1.1: Tetraspanin construction.Tetraspanin protein construction consists of 200-350 amino acids. It comprises of four transmembrane protein domains attach to two different sizes of extracellular cringles and one intracellular cringle. The little extracellular cringle, EC1, made up of 32-53 amino acids while the big extracellular cringle, EC2 made up of 105-209 amino acids. This construction is flanked by short N-terminal and C-terminal cytoplasmic dress suits. |
- Structure and map of CD63
Tetraspanin CD63 ( besides known as LAMP-3, LIMP-1, ME491 or Pltgp40 ) was the first characterized tetraspanin derived from the early phases of human melanoma tumor cells ( Hotta et al. 1988 ) . The presence of 6 cysteine residues in the EC2 and conserved polar residues in the transmembrane domains differentiate CD63 from the other tetraspanins ( Hemler, 2003 ) . Until now, CD63 protein is found to be expressed ubiquitously on all types of mammalian cell that have been studied ( Pols and Klumperman, 2009 ) . It is localized at the cell surface and due to the C-terminal lysosomal aiming motive, it present copiously in lysosomal membranes and intraluminal cysts ( ILVs ) of multi vesicular organic structures ( MVBs ) within the endosomal system ( Pols and Klumperman, 2009 ) . Upon cell activation and secernment, merger of MVBs with the plasma membrane will let go of the ILVs to the extracellular infinite which so termed as exosomes ( Pols and Klumperman, 2009 ) . Trafficking of intracellular CD63 to the cell surface has made the CD63 as an activation marker of several haematopoietic cells ( Lettau et al. 2007 ) while surface exposure of CD63 on basophils map as degranulation and diagnostic marker in allergic diseases ( Ebo et al. 2002 ; Sturm et Al. 2004 ; Eborlein-Konig et Al. 2006 ) .
Like any other tetraspanins, CD63 interacts with diverse type of proteins including other type of tetraspanins, cell surface receptors, kinases and adapter proteins ( Pols and Klumperman, 2009 ) . Within TEMs, CD63 was found to be interacting straight with integrins, syntenin-1 and membrane metalloproteases ( Latysheva et al. 2006 ; Jung et Al. 2008 ) therefore it is believed that CD63 might modulate or be involved in cell adhesion, motility and phagocytosis ( Yoshida et al. 2008 ) .In vitrosurvey in the 1990 found that culturing the myeloid cell lines with the human CD63 monoclonal antibody ( mAb ) , anti-hCD63 ( antecedently known as MAb 710F ) increase the spreading of monocytic cells and do them adhere strongly to the serum-coated plastic ( Koyama et al. 1990 ) . However, the same antibody was found to impair the adhesion of neutrophilic granulocytes to the pre-treated endothelium ( Toothill et al. 1990 ) . Then once more, rapid internalisation of hCD63 along with enhanced motility among dendritic cells was observed utilizing different anti-hCD63 ( Manteggazza et al. 2004 ) . On the other manus, mAb targeted against EC2 of CD63 ( named D545 ) was found to change the spreading of thrombocyte ( Israels and McMillan-Ward, 2005 ) .
In vivo, CD63 smasher mouse showed a mild phenotype where there was altered H2O balance by the kidney and GI piece of land, while the lamellar inclusions were abnormally accumulated in the rule cells of the roll uping canal ( Schroder et al. 2009 ) . However, a strong phenotype displayed in the CD63 cistron smasher zebrafish which the embryos treated with morpholinos aiming CD63 were failed to hatch ( TrikiA‡ et al. 2011 ) . This damage might due to the deficient of secreted proteolytic enzymes in the hatching secretory organ which are required for chorion-softening ( TrikiA‡ et al. 2011 ) . In add-on, there was cellular morphology disorganization of the hatching secretory organs in thisin vivotheoretical account at the cellular and intracellular degrees ( TrikiA‡ et al. 2011 ) .
- CD63 in the Fca¶“RI-mediated mast cell degranulation
The association of tetraspanin CD63 with the high affinity IgE receptor, Fc epsilon receptor I ( Fca¶“RI ) on the mast cell or basophil has been proven in a figure of surveies ( Kitani et al. 1991 ; Smith et Al. 1995 ) . To analyze the signalling via this receptor, the rat basophilic leukemia cell line ( RBL-2H3 ) is extensively used asin vitrotheoretical account because of the similar features portion with the mast cells. A survey by Kitani et Al. ( 1991 ) has demonstrated that anti-rat CD63 mAb efficaciously suppress IgE-mediated degranulation, nevertheless, in the other survey, hCD63 antibodies are able to trip secernment in the CD63 transfected RBL-2H3 cells ( Smith et al. 1995 ) .
Antibodies against the tetraspanins including CD63 are capable in modulating the degranulation because the tetraspanins are thought to be interacting so modulating the signalling bunch which involve in cell adhesion and cell spread mechanism that enhance the IgE-mediated secernment in the RBL-2H3 cell line ( Higginbottom et al. 2000 ) . Subsequently, this was confirmed by a survey in 2005 which mAb directed against CD63 inhibits mast cell adhesion to fibronectin and vitronectin and suppress the Fc?RI-mediated degranulation ( Kraft et al. 2005 ) . Additionally, the suppression of the degranulation merely happened in the cells that adhere to the ECM proteins ( Kraft et al. 2005 ) . To back up this, Gab2-PI3K-PKC? signaling tract that is known to be critical for degranulation every bit good as adhesion besides found to be suppressed by anti-CD63 ( Kraft et al. 2005 ) .
In 2010, a fresh farinaceous discrepancy of tetraspanin CD63 was found to be an sole marker of degranulated human mast cell ( Schafer et al. 2010 ) .In vitro, the human mast cell was found to be underwent perennial rhythms of Fc?RI-mediated degranulation and interestingly, antibodies against this CD63 discrepancy impaired these rhythms ( Schafer et al. 2010 ) . Therefore, it is thought that this discrepancy might affect in bit-by-bit degranulation, a procedure which sustains and aggravates allergic responses, because it is assumed that partially degranulated human mast cellsin vivocan remain at the activation site and magnify subsequent response in the presence of another antigen ( Schafer et al. 2010 ) .
Recently,in vivosurvey utilizing CD63 smasher mouse theoretical account ( MC-deficient Kitw/w-v) found that there was a important lessening in Fc?RI-mediated degranulation in the mast cell derived from the bone marrow of this smasher mouse ( Kraft et al. 2013 ) . Hence, this reflects that in the absence or suppression of CD63, thein vivoacute allergic reactions can be reduced and made the tetraspanin CD63 as a important constituent in the allergic redness ( Kraft et al. 2013 ) .
- Undertaking background
A mutated signifier of CD63 which lacks N-linked glycosylation at amino acid 172 in the EC2 of the protein named CD63N172A (Figure 1.2) has been antecedently generated by the lab ( Gorakh Mal, PhD Thesis, University of Sheffield, 2005 ) .
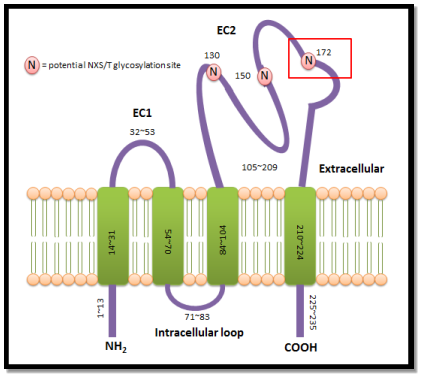 |
| Figure 1.2:Potential glycosylation sites of CD63.There are three possible glycosylation sites at the big extracellular cringle, EC2. Variant signifier of tetraspanin CD63, CD63N172A which has been generated antecedently is deficiencies of N-linked glycosylation site at the amino acid 172. |
We believe that this CD63 discrepancy likely tally to the late described variant signifier by Schafer et Al. 2010. CD63N172A displays unusual look compared to the wild type of CD63 ( CD63wt ) (Figure 1.3 and 1.4) . The preliminary informations that have been obtained so far besides suggested that the IgE-mediated degranulation response is altered in RBL-2H3 cells transfected with this mutated CD63. To supply the clear apprehension of the function of CD63 in the degranulation response with relevancy to allergy, this undertaking aims to further compare the differences of IgE-mediated degranulation between CD63N172A and CD63wt. The surface look of CD63N172A, CD63wt and Fca¶“RI will besides be monitored and the possible differences in the trafficking will be investigated. Finally, this undertaking aims to analyze the associations of both, CD63N172A and CD63wt with the, Fca¶“RI.
| CD63wt
|
CD63N172A
|
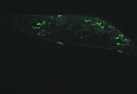
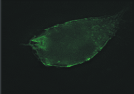
| Figure 1.3 Preliminary informations – cell surface immunofluorescence staining.Cell surface look of CD63wt and CD63N172A viewed by fluorescence microscope. Due to the C-terminal lysosomal aiming motive, tetraspanin CD63 copiously present in late endosomal compartment. However, mutant of N-linked glycosylation site at the amino acid 172 cause the protein to expressed more entirely on the cell surface. | |
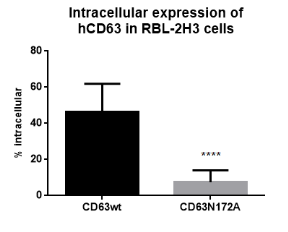 |
| Figure 1.4Preliminary informations – intracellular immunofluorescence staining. Using the odd t-test, this preliminary information shows that there was important different of intracellular look between the wild-type of CD63 and the mutant signifier of CD63 ( p=0.0001 ) . CD63wt is widely expressed inside the cell while merely a few per cents intracellular look of CD63N172A. |
- Hypothesis
Nitrogen172A mutant signifier of CD63 is hypothesized to be corresponds functionally to a discrepancy of this protein which occurs of course. We besides hypothesized that over look of CD63N172A alters the mast cell degranulation response upon IgE cross-linking, possibly by modifying the trafficking of the Fca¶“RI.
- Plan of probe
The RBL-2H3 cell line is used as a mast cell theoretical account in this undertaking. Transfectants that stably express wild type CD63 and N172A mutant signifiers of hCD63 have already been generated by the lab antecedently ( Gorakh Mal, PhD Thesis, University of Sheffield, 2005 ) . IgE-mediated degranulation response will be assessed by ?-hexosaminidase release assay utilizing colorimetric technique. The add-on of the IgE will stimulated the cells and different concentration DNP-HSA will be added to do the IgE cross-linking. The surface look of CD63wt, CD63N172A and the Fca¶“RI will be monitored utilizing specific antibodies and flow cytometry ( FACS ) analysis. The possible differences between CD63wt and CD63N172A in Fca¶“RI trafficking besides will be investigated utilizing specific antibodies and FACS analysis. To look into the associations of the Fca¶“RI with CD63wt and CD63N172A, “pull-down” experiments will be carried out by immunoblotting.