Introduction
Proteins are biological supermolecules composed of amino acids. Proteins consist of one or more polypeptide which are the concatenation of aminic acids interconnected by peptide bonds. Alberts et al. , ( 2013 ) detailed that, aminic acids of proteins is either hydrophobic or hydrophilic in nature. Therefore the ensuing polypeptide concatenation shows an amphipathic feature. Hydrophilic aminic acids exist peripherally in some biological system and they are extremely H2O soluble. Whereas some aminic acid does non be the polar groups to the environment.
‘’The most of import factors that influence protein solubility are construction, size, charge and the solvent‘’ (Burgess, and Deutscher, 2009). Besides Burgess, and Deutscher ( 2009 ) stated that one time the precipitation obtained, the solution can be separated by centrifugation or precipitation.
‘’Protein precipitates are sums of protein molecular big plenty to be seeable and to be collected by centrifugation. The distribution of hydrophilic and hydrophobic residues at the surface of a protein determines its solubility properties‘’ . (Rosenburg, 2006) .Precipitation is chiefly done for dressed ore the mark protein. And it is attained by adding reagents such as salts (ammonium sulphate) or organic dissolvers (propanone or ethyl alcohol). (Hatti-Kaul and Mattiasson, 2003)
Isolation of Casein
Milk contains three sorts of proteins: caseins, lactalbumins, and lactoglobulins, all of which are ball-shaped proteins. ( Spurlock, 2014 ) . Ahluwalia and Dhingra, ( 2005 ) stated that, Casein is a combination of phosphoproteins showing in milk and cheese.it is bing to the sum of 3 % in milk along with 4-5 % of lactose and 3-4 % of fats and the remainder is H2O. Caseins exist in micelles which are composed of bomber micelles linked by the feature of hydrocolloid which are freely suspended in the aqueous stage of milk. ( Tarte, 2009 ) . Casein can be electrophoretically fractioned into four major constituents: alpha- , beta- , gamma-and kappa- casein. Casein develops precipitation from milk at pH 4.6, which has a negative charge when comparison to the pH of the milk. Therefor it can be hasty as salt by adding acids. ( Miller, Jarvis and McBean, 2006 ).
Aims
To learn the methods of protein precipitation and to associate the solubility of protein with its construction. To learn the methods of isolation of casein from milk and to find the per centum of casein presented in the ( powdered ) milk.
Materials
- Test tubing
- Beakers
- Pipet
- Clamp
- Filtering paper
- Electronic balance
- Watch glass
- Bunsen burner
- Albumin sample
- Ammonium sulphate
- Sodium hydrated oxide
- Copper sulphate
- Ethyl alcohol
- Picric acid
- Lead nitrate
- Powdered milk
- Warm H2O
Precipitation by Salts
Albumin, 3.00ml was taken into a trial tubing, ammonium sulphate was added to it and was mixed until the solution gets saturated. The solution was allowed to stand for approximately 5 proceedingss and filtered by utilizing filter paper. The biuret trial was done to the filtered solution. 3.00 milliliter of filtered solution was taken into another trial tubing and same sum of NaOH was added to it, CuSO4 was added bead by bead.
Precipitation by Organic Dissolvers
Albumin, 1.00 milliliter was taken into a trial tubing utilizing a pipette. And 4.00 milliliter of ethyl alcohol was added.the solution was assorted good and was allowed to stand.
Precipitation by Acidic Agents
Picric acerb solution, 1.00 milliliter was added into 1.00ml of albumin solution.
Precipitation by Heavy Metal Ions
Lead nitrate, 8 beads were added into 1.00 milliliter of albumen.
Precipitation by Heat and Acid
Albumin, 10 milliliter was taken into a trial tubing and the upper portion of the solution was held over the Bunsen fire. After the observation few beads of 1 % acetic acid were added.
Isolation of Casein
Powdered milk ( non-fat ), 17.5 g was weighed by utilizing electronic balance and was dissolved by adding 62.5 milliliter of warm H2O in a 200ml beaker. Acetic acid ( 10 % ) was added in a bead wise mode with stirring until the liquid alterations in to clear solution. the obtained solution was filtered by utilizing clinch, filtrating stuff and beaker. The output casein was allowed to dry and was weighed utilizing electronic balance. Biuret trial was done for the filtered solution. 3.00 milliliter of filtered solution was taken into another trial tubing and same sum of NaOH was added to it, CuSO4 was added bead by bead.
Consequences
Trial |
Observation |
Intervention |
| Precipitation by metal ions | White colour precipitation | Proteins can be precipitated by metal ions ( positive for proteins ) |
| Precipitation by heat and acid | Initially nebulose white precipitation was observed on the upper portion of the solution and by adding acetic acid white colour precipitation was observed. | Proteins can be precipitate by heat and acid ( positive for proteins ) |
| Precipitation by organic dissolvers | White colour precipitation was observed | Proteins can be precipitate by organic dissolvers ( positive for proteins ) |
| Precipitation by acidic agent | White colour precipitation was observed | Proteins can be precipitate by acidic agents. ( positive for proteins ) |
| Precipitation of salts
Biuret trial |
White colour precipitation was observed.
Purple colour ring was observed
|
Proteins can be precipitate by salts.
Positive for proteins. |
| Isolation of casein
Biuret trial for filtration |
Casein 13.01g was weighed
Purple colour ring was formed in filtered casein solution |
Output % =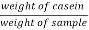 ? 100 ? 100
= = 74.30 % Positive for proteins. |
Discussions
Precipitation of protein can be obtained by isoelectric precipitation method. ‘’isoelectric precipitation is the most widely used method’ ( Fox and McSweeney, 2003 ) . Proteins can be precipitated by conveying their pH to its isoelectric point in which protein solubility is really low. ( Shankara, 2008 )
Proteins can be precipitate by salts in two ways, half impregnation with ammonium sulphate and full impregnation with ammonium sulphate. Rashmi, ( 2002 ) stated that, different proteins show different precipitation reaction towards diverse agents. The full impregnation with ammonium sulphate was done in the research lab. Besides the filtrate was tested by biuret reagent, resulted violet colour. ‘Compounds with two or more peptide bonds give a violet colour with alkalic Cu sulfate’ ( Rashmi, 2002 )
Proteins are strong in solution when they are enclosed by wholly hydrogen-bonded H2O molecules, as H2O molecules with extra H adhering ability have greater information and are more aggressive. ( Chaplin, 2014 ) hydrated sphere lessening the non -polarity. Higher the diameter of the domain higher the solubility. For an illustration, it is easy to precipitate globulin from proteins by adding salts, than albumin because globulin has little diameter of hydrated domain when comparison to albumin.
The similar construct is used in precipitating proteins by organic dissolvers and acidic agents. Organic dissolvers take the hydrated domain and lessening solubility ensuing increase precipitation. Acids neutralize the mutual opposition of the hydrated domain and lessening solubility in order to increase precipitation.
Denaturation occur on warming or adding acidic agents to proteins. Therefor its alteration the mutual opposition of a protein by altering the agreements of polar and non-polar groups within the molecule. Less mutual opposition lessening the solubility and increases the precipitation.
Precipitation by heavy metal ions lead nitrate was used alternatively of lead ethanoate or mercurous nitrate. Shankara ( 2008 ) stated that, metal ions which are positively charged interrelate with negatively charged groups of the protein bring forthing precipitation as metal-proteinate composite.
Harmonizing to the per centum of output and from the consequence of biuret trial of the filtrate, there can be some proteins present in the filtrate. Because, the milk contains about 3.5 % protein by weight and of the entire protein, approximately 80 % is casein and 20 % is whey protein. ( Miller, Jarvis and McBean, 2006 )
Filtration of casein can be done in two ways. Such as, gravitative filtration and sucktional filtration.
Decision
Proteins were precipitated by utilizing metal ions, heat, organic dissolvers, acidic agents and salts.
The per centum of output casein of the sample is 74.30 % .
References
- Ahluwalia, V. and Dhingra, S. ( 2005 ) .College Practical Chemistry.[ Online ] Google Books. Available at:books.google.lk/books? id=1OgRECl_nw
- Prince alberts, B. , Bray, D. , hopkin, K. , Johnson, A. , Lewis, J. , Raff, M. , Roberts, K. and Walter, P. ( 2013 ). Essential Cell Biology, Fourth Edition. [ Online ] Google Books. Available at: books.google.lk/books? id=Cg4WAgAAQBAJ
- Burgess, , R. and Deutscher, M. ( 2009 ). Guide to Protein Purification. [ Online ] Google Books. Available at: books.google.lk/books? id=f6Lp4yna4ho
- Chaplin, M. ( 2014 ) . Home | London South Bank University. [ Online ] Www1.lsbu.ac.uk. Available at: www1.lsbu.ac.uk/
- Fox, P. and McSweeney, P. ( 2003 ) .Advanced Dairy Chemistry: Volume 1: Proteins, Parts A & A ; B.[ Online ] Google Books. Available at: books.google.lk/books? id=RMNkAc5PkVE
- Hatti-Kaul, R. and Mattiasson, B. ( 2003 ) .Isolation and Purification of Proteins. [ Online ] Google Books. Available at: books.google.lk/books? id=CdHn45QCU_8
- Miller, G. , Jarvis, J. and McBean, L. ( 2006 ) .Handbook of Dairy Foods and Nutrition, ThirdEdition.[ Online ] Google Books. Available at: books.google.lk/books? id=5tleQ0aLJvo
- Rashmi, J. ( 2002 ) .A Textbook of Practical Biochemistry. [ Online ] Google Books. Available at: books.google.lk/books? id=wBfAshZ3ZaU
- Rosenburg, ( 2006 ) .Protein Analysis and Purification. [ Online ] Google Books. Available at: books.google.lk/books? id=gi-UgCF8G6E
- Shankara, ( 2008 ) .Practical Biochemistry 2008. [ Online ] Google Books. Available at: books.google.lk/books? id=nPphGdHO_Xc
- Spurlock, D. ( 2014 ) .Deborah Spurlock ‘s Chemistry Home page. [ Online ] Homepages.ius.edu. Available at: homepages.ius.edu/DSPURLOC/
- Tarte , R. ( 2009 ) .Ingredients in Meat Merchandises. [ Online ] Google Books. Available at: books.google.lk/books? id=C-wrQaaXxj0
- Walsh, G. ( 2002 ) .Proteins.[ Online ] Google Books. Available at: books.google.lk/books? id=EXTEjL2wTnY






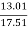 ? 100
? 100