Introduction
Battery is defined as a device that produces electricity. The electricity produced by battery is normally used to provide energy to electronic devices such as ticker, camera, wireless and others. Battery supplies energy to these devices through the transition of stored chemical energy into electric energy. Batteries come in assorted sizes and forms. They are besides categorized into a few classs. The classs of batteries are primary batteries and secondary batteries. The primary batteries consist of non-rechargeable batteries which can merely be used one time and so discarded while the secondary batteries consist of rechargeable batteries that can be discharged and recharged for multiple figure of times. The tabular array below shows the illustrations of batteries harmonizing to their classs.
| Primary batteries | Secondary batteries |
| Alkaline battery
Dry cell Galvanic cell Lithium battery Mercury battery Weston cell |
Lead-acid battery
Lithium-ion battery Nickel-cadmium battery Rechargeable alkaline battery |
Table 1: Examples of batteries in their classs

Image 1: Examples of different sizes and forms of batteries ( beginning: Wikipedia.com )
Batteries are being used a batch in our day-to-day life. They are being used both by the family and the industry. A batch of the family appliances that we use mundane usage batteries to run. Some of them are our wireless, dismay clock, camera and our French telephone. Meanwhile, some of the industries that applied battery in their merchandises are the automotive industry and electronic devices industry. Cars produced by the automotive industry depend on battery for some of its constituents to work decently. Furthermore, there are some electric autos that are to the full operated by rechargeable batteries in an effort to cut down the use of fossil fuels.
The use of battery came with a few jeopardies. Some of the illustrations of jeopardies that came with battery use are jeopardies, of detonation, escape and toxic stuffs. A battery could detonate if they are misused of malfunctioned. One of the few illustrations of abuse of a battery is when person is trying to reload a non-rechargeable battery. This action would take to the happening of short-circuit that will take to the detonation of the battery. Rechargeable battery will besides detonate if it is over-charged. Another jeopardy that came with the use of battery is leakage. Battery contains assorted chemicals that are either caustic or toxicant or both. Therefore, it is a really unsafe happening to come into contact with the leaked chemicals from the battery. The image below shows the image of a leaked alkaline battery.

Image 2: Leaked alkalic battery ( beginning: Wikipedia.com )
Last but non least, the jeopardy that came with the use of a battery is the toxic stuffs that it contains. Most of the batteries contain really unsafe chemicals such as heavy metals. Some of the illustrations of heavy metals that are contained in a battery are lead, nickel, Cd and quicksilver. It is a well-known fact that exposure to these sort of heavy metal is damaging to the human wellness. Exposure towards lead could do assorted diseases particularly to yearlings. Meanwhile, exposure to mercury could take to human death.
From the above grounds, it can be seen that batteries are toxic and corrosive ; which is two of the traits used to sort a stuff as risky waste apart from being flammable and reactive. Due to these grounds, battery is being categorized as one of the risky waste produced by the family ( Inglezakis & A ; Moustakas, 2015 ) .
DISPOSAL OF BATTERIES
Presents, people are utilizing more and more household batteries. Most of electronic devices uses batteries. For illustrations, manus phones, torchlight, ticker, clock, reckoners, playthings, cameras, and many more. Batteries that we are utilizing contain heavy metals for examples quicksilver, lead, Cd and besides Ni which when it is non fain decently, can pollute the environment. When incinerated, the ash produced by the burning procedure might be released into the air and may do air pollution. Heavy metals in the landfill may hold the possible to ooze easy into the dirt, groundwater and even surface H2O. During the combustion procedure, heavy metals such as quicksilver will get away and zap into the air.
Batteries that are non disposed decently may do jeopardies and other possible jobs, for illustrations:
- It may foul Waterss and rivers because the metals will zap into the ambiance when it is burned.
- It will lend to heavy metals that have possible to leach the solid waste landfills.
- Lead and acid that come from the batteries may potentially foul the environment and Waterss.
- The batteries contains strong caustic acids.
- It gives jeopardies to eyes and tegument and besides may do Burnss.
Household Batteries
Normally, there are two types of batteries which are known as primary and secondary which besides known as rechargeable batteries. Primary batteries are batteries that can non be reused. Primary batteries include alkaline/manganese, carbon-zinc, mercuric-oxide, zinc-air, silver-oxide, and other types of button batteries. On the other manus, secondary batteries are batteries that is rechargeable and can be reused many times. Secondary batteries include lead-acid, nickel-cadmium, and potentially nickel-hydrogen.
Typical types of family batteries
| Primary Batteries | Common utilizations |
| Alkaline | Cassettes participants, wirelesss |
| Carbon-zinc | Flashlight, plaything |
| Lithium | Cameras, reckoners, tickers, computing machines |
| Mercury | Hearing AIDSs, pacesetters, cameras, reckoners, tickers |
| Silver | Hearing AIDSs, tickers, cameras, reckoners |
| Zinc | Hearing AIDSs, beepers |
| Secondary Batteries | Common utilizations |
| Nickel-cadmium | Cameras, rechargeable contraptions such as portable power tools, handheld vacuity |
| Small sealed lead-acid | Camcorders, computing machines, portable wirelesss and tape participants, cellular phones, lawn mower starting motor |
Today’s patterns of disposal of batteries
Presently, consumers will freely dump the used batteries in their refuse bin. This is because they have the trouble to place the types of batteries. Some of the consumers will throw the used batteries anyplace without cognizing the consequence of the batteries to the environment.
Suggestion on proper disposal of batteries
Duanet Al. had stated that there are really small inside informations sing the recovery of family risky waste such as lead-acid battery. Furthermore, some of the equipment and engineering are traditional and hence lack the installations to ease environmental protection while retrieving the waste. This will finally take to serious environmental jobs. Therefore, we would wish to propose for the authorization to get down raising consciousness of the importance of dividing battery waste from the common family waste. At present, the battery waste is being discarded along with the common family waste. It is merely when the waste reached the recovery installations of the landfill that it is being separated from the common waste. If we separate the battery waste from the other waste at beginning, we will salvage the clip and the work force needed to divide the battery waste and therefore will cut down the hazard of the battery waste being improperly discarded to the landfill alternatively of being sent to the proper installations to handle risky waste.
- Alkaline batteries can non be disposed in the regular rubbish. They must travel with family risky waste.

Image 3: Alkaline battery ( beginning:hypertext transfer protocol: //www.wikihow.com/Dispose-of-Batteries )
- Button-shaped batteries must be brought to risky waste aggregation site to dispose.

Image 4: Button-shaped battery ( beginning:hypertext transfer protocol: //www.wikihow.com/Dispose-of-Batteries )
- Lithium and lithium-ion batteries must be disposed at a battery recycling centre.
Image 5: Lithium and lithium-ion battery ( beginning:hypertext transfer protocol: //www.wikihow.com/Dispose-of-Batteries )
- Rechargeable sealed lead-acid or nickel-cadmium batteries must be disposed at a family risky waste site. They may besides can be taken to a recycling Centre.

Image 6: Lead-acid battery ( beginning:hypertext transfer protocol: //www.wikihow.com/Dispose-of-Batteries )
- Lead-acid vehicles batteries must be disposed at the retail merchant. This batteries contain really caustic stuffs.

Image 7: Lead-acid vehicle battery ( beginning:hypertext transfer protocol: //www.wikihow.com/Dispose-of-Batteries)
Summary on Real Disposal and Recycling Method
| Battery Types | Proper Disposal |
| Alkaline ( manganese ) | Dispose in the refuse ( normal municipal waste ) |
| Button-shaped batteries | Dispose to household risky waste aggregation site |
| Carbon Zn | Dispose in the refuse ( normal municipal waste ) |
| Lithium and lithium-ion batteries | This type of batteries can be recycled |
| Nickel-cadmium | Dispose to household risky waste aggregation site |
| Nickel Metal Hydride | Can be disposed to normal municipal waste watercourse and besides this type of batteries can be recycled |
| Reuseable Alkaline Manganese | Dispose in the refuse |
| Sealed-lead acid | Dispose to household risky waste aggregation site |
| Lead-acid vehicle batteries | Dispose at the retail merchant or to the metal recycler in which they may pay you back for the vehicles battery that you dispose |
| Silver oxide | This type of batteries may be acceptable for recycling |
TREATMENT FOR HOUSEHOLD BATTERY WASTE
As the engineering become extremely advanced, with most of electronic contraptions rely on batteries for primary power beginning, the demand for battery supplies has quickly increased worldwide ( D. Ra, 2006 ) . To back up the statement before, the ingestion of batteries in USA and Europe is statistically estimated about 8 billion unit yearly ( A.M. Bernardes, 2004 ) . In Japan, 6 billion batteries were manufactured in 2004 interim in Brazil ; record has proved that 1 billion units of batteries are consumed per twelvemonth ( A.L. Salgado, 2003 ) .
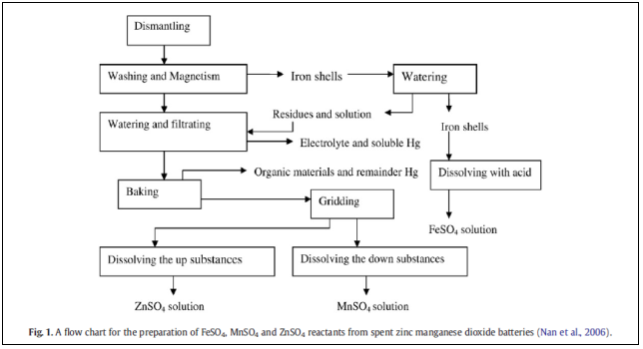
Recycling batteries is comparatively critical because toxic elements or compound in batteries may leak into the land and impact the dirt food if disposal is non decently done. Since batteries represent valuable waste stuff for the recovery of metals present or their compound, recycling of used batteries could ensue in economic net incomes. The recycling procedure should diminish bit volume, divides battery constituents into specified affair and decrease the impact of batteries towards the environment ( De Souza and Tenorio, 2004 ) . In order to recycle batteries waste, several important stairss are needed to retrieve and obtain the metal component from the battery itself.
- Physical procedures
- Chemical procedures
PHYSICAL Procedure
Normally, the physical recovery processes consist of figure of chronological stairss from screening, magnetic separation, leveling and crunching. These pre-treatment activities are indispensable in order to heighten metal disintegration rates in the aqueous stage ( Salgado et al. , 2003 ) .
In the sorting measure, alkaline, zinc-carbon and lithium-ion batteries are separated consequently to insulate the cathode stuffs from other stuffs. Following, the leveling measure requires filtration of battery pulverization consisting of a mixture of black lead and metallic oxide from Fe garbages, paper and plastic. The metallic garbages, for case, could be recycled by a pyrometallurgical intervention. The concluding measure in pre-treatment procedure is crunching. Ball factory is used to crunch all the battery waste into smaller atom size accordingly better the efficiency of the leaching measure ( Veloso et al.,2005 ) .
CHEMICAL PROCESS
Pyrometallurgical procedures
Pyrometallurgical procedures are the most conventional procedure used in the recycling of batteries even it is rather dearly-won. Basically, it involves volatization of selective metals at highly high temperature and condensation procedure ( Salgado et al. , 2003 ; De Michelis et al. , 2007 ; Li and Xi, 2005 ) . Pyrometallurgical intervention should give pure metals or intercede compound that is marketable and give high value, for illustration oxides of Fe, Cu, Zn, Cr, Sn, manganese.
| Battery waste | Temperature ( ?c ) | Metallic element recovered |
| Ni–Cd batteries | 900 °C | Pure Cd |
| Zinc–carbon batteries | 1500 °C | Zinc and quicksilver |
| Zn–Mn batteries | 800 °C | Pure Mn |
There are four degrees of pyrometallurgy in usage today, which differ in both the sum of heat applied and the types of reactions which result.
- Calcining
Calcination is a warming processes that occur in the presence of O to divide the volatile compounds from the original affair, where it originate a stage of targeted mineral to be farther decompose. At this degree, each of volatile crystalline H2O in bauxite and gypsum is evaporated going gaseous that taken topographic point in specialised furnaces ( rotary kilns, shaft furnace ) . For an illustration is decomposition of hydrates such as ferrous hydrated oxide to ferric oxide and H2O vapour.
- Roasting
Roasting procedure is the procedure for purification of metals with hot air through a thermic gas-solid reaction. Generally, sulfide minerals are the compound treated whereby it will change over into an oxide as S dioxide gas is released. This thermic gas-solid reaction includes series of other reactions such as oxidization, decrease, chlorination and pyrohydrolysis. This purification procedure must be handled in a really good contained country as it will let go of Numberss of unsafe gaseous, including arsenic, antimony and lead.
- Smelting
Smelting is the most commonly-known pyro metallurgical technique, and is by and large used to separate metals from their ores. In this technique, the warming procedure will cut down at least one of the elements to a liquefied stage. Every now and so, metal oxides will be smelted utilizing cut downing agent such as coke or wood coal. This is done to organize C dioxide to assist the emanation of O molecules from the ore. At other times a flux is added, which both catalyzes desired reactions and so chemically binds with the drosss or gangue in the ore to organize scoria, a waste merchandise. Some types of electronically-induced chemical transmutations, such as the decrease of aluminium oxide into aluminium fluoride, are besides referred to as smelting.
- Polishing
The last but non least procedure is polishing, where it is differ from the other methods as it does non affect any chemical change of the targeted metal. The procedure burns off all the drosss in the metal but in the interim, it refines and purifies the metal. For illustration, pull outing Ag from lead is one of the common refinement processes done. The metal, Fe is smelted at early phase and refined to extinguish the staying 4-5 % C and besides Si after smelting procedures. The refinement procedure will increase the pureness of cured metal if it is done repeatedly.
Hydrometallurgical procedures
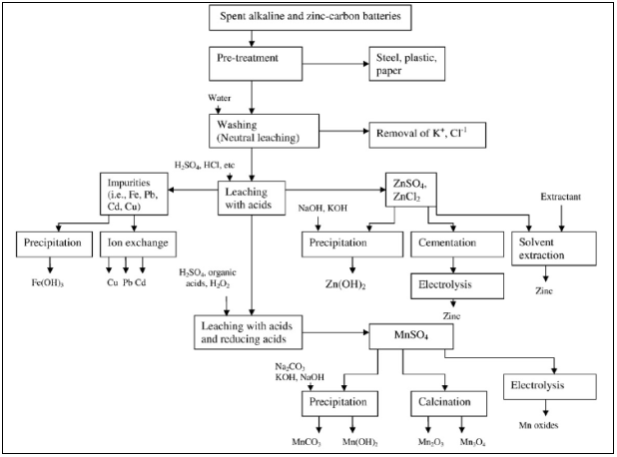
This well established method of retrieving metal from natural stuff is preferred by much company as it is efficient and cut their cost to run. It is selected as an extraction procedure every bit good as an environmental control since it does non bring forth mush waste. In general, hydrometallurgical procedures consist of different stairss of pre –treatment for different type of battery waste followed by leaching and metal separation.
It has more benefits as compared to pyrometallurgical procedure. Hydrometallurgical procedure is cheap to run, probably to retrieve leachants after procedure and besides do non present any air pollution as there are no atoms produced. However, some pre-treatment stairss are required in order to better metal disintegration rates in the aqueous stage, like battery categorization, leveling, magnetic separation, and leaching ( Salgado et al. , 2003 ; Karakaya et al. , 2007 ) . The process will take longer period and affect in assorted chemical ingestion.
Toro et Al. ( 2006 ) developed a hydrometallurgical procedure for the recycling of all cell constituents utilizing sulphuric acid leaching with waste saccharides as cut downing compounds. The hydrometallurgy procedure comprises the undermentioned phases:
- Leaching
- Ion exchange
- Chemical precipitation
- Electrowinning
- Leaching
Leaching activity comprises the reaction of stuff with a valuable metal with aqueous solutions incorporating a lixiviant. Lixiviant solution occurs of course in both, acidic and basic. The type and concentration of lixiviant is normally controlled depending on the metals that are to be recovered. Some of the of import parametric quantities in leaching include temperature, pH of solution and oxidization potency. All of them are frequently manipulated to maximise disintegration of targeted metal into aqueous stage.
- Ion exchange
At this degree, chelating agent such as natural zeolite, activated C, rosins and organic liquid will be used to soak the mixture. The chelating agent will respond with targeted metal for cations and anions exchange procedure to happen.
- Chemical precipitation
Compared to liquid–liquid extraction, precipitation is a cheaper and simpler method for hydrometallurgy. In this procedures, chemical precipitation is needed to sublimate solution from any other drosss such as Fe, Cu, Cd and so on. Precipitation itself has a series of stairss needed to be followed so that any species would non transcend its bound of solubility.
- Electrolysis
Electrowinning and electrorefining are both process needed to be done under electrolysis of hydrometallurgy. Respectively, both of it would retrieve and sublimate the targeted metals utilizing electro deposition procedure at cathode. Meanwhile, at anode, it is either metal disintegration or viing oxidation reaction to finish the electrolysis process.
Mentions
- Duan, H. et al. , 2008. Hazardous waste coevals and direction in China: A Review.Journal of Hazardous Materials, 158, pp.221-27.
- Inglezakis, V.J. & A ; Moustakas, K. , 2015. Household risky waste direction: A reappraisal.Journal of Environmental Management, 150, pp.310-21.
- A.L. Salgado, A. V. ( 2003 ) .Power Beginnings 115, 367–373.
- A.M. Bernardes, D. E. ( 2004 ) .Power Beginnings 130, 291–298.
- Bernardes, A.M. , Espinosa, D.C.R. , Tenorio, J.A.S. , 2004. Recycling of batteries: a reappraisal of
- current procedures and engineerings. Journal of Power Sources 130, 291–298.
- D. Ra, K. H. ( 2006 ) . Power Sources 163. 284–28.
- De Souza, C.C.B.M. , Tenorio, J.A.S. , 2004. Coincident recovery of Zn and manganese dioxide from family alkaline batteries through hydrometallurgical processing. Journal of Power Sources 136, 191–196.
- De Souza, C.C.B.M. , Oliveira, D.C. , Tenorio, J.A.S. , 2001. Word picture of used alkaline batteries powder and analysis of Zn recovery by acid leaching. Journal of Power Sources 103, 120–126.
- Hanulik, J. , 2000. Procedure for the recycling of batteries, particularly dry batteries. United States Patent, Patent Number: 6,009,817.
- Hurd, D.J. , Muchnick, D.M. , Schedler, M.F. , 1993. Recycling of dry cell batteries, Pollution Technology ( reexamine figure 213 ) , Noyes Data Corporation.
- Ismail, A.A. , Ali, E.A. , Ibrahim, I.A. , Ahmed, M.S. , 2004. A comparative survey on acid
- leaching of low class Mn ore utilizing some industrial wastes as reducing agents. The Canadian Journal of Chemical Engineering 82, 1296–1300.
- Jha, M.K. , Kumar, V. , Singh, R.J. , 2001. Review of hydrometallurgical recovery of Zn
- from industrial wastes. Resources, Conservation and Recycling 33, 1–22.





