The Effect of Molecular Weight to the Diffusion Rate of Substances[ 1 ]
The consequence of molecular weight to the rate of diffusion of substances had been obtained through agarose-water gel experiment. Drops of the undermentioned dye samples were used as controlled variables: K permanganate, K bichromate and methylene blue. Each dye has a molecular weight of 158 g/ mol, 294 g/mol and 374 g/mol, severally. The diameter of the aforesaid variables were measured every 180 seconds for 30 proceedingss. As an result, K permanganate had the highest mean rate of diffusion with a value of mm/min. , K bichromate had 4.13 mm/min and methylene blue had mm/min. which was considered as the lowest among the three variables. Hence, molecular weight straight affects the rate of diffusion of a certain substance.
Introduction
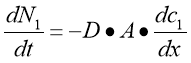
Diffusion — normally defined as the motion of atoms from a part of higher concentration to a part of lower concentration, is merely one of the many procedures due to the kinetic belongingss of molecules. It was Robert Brown, a celebrated English phytologist who observed the motion of such atoms through looking at the pollen grains suspended in H2O with the assistance of a microscope. Diffusion is resulted by this random gesture of atoms, called as the Brownian motion ( Dean, 1948 ) . Through this motion, atoms spread out, with a gradual lessening in the concentration gradient until the atoms reach the province of equilibrium. Therefore, the atoms are drastically diffused throughout. The chief definition of diffusion was clearly demonstrated by Adolf Fick in 1855 through a mathematical equation:
The equation above is known asFick’s first jurisprudence of diffusion, wherein dN1/dt is the rate of atoms go throughing through a certain plane, District of Columbia1/dx is the concentration gradient, A is the plane country and D is the diffusion coefficient. The negative mark beside D shows that the way of flow is contrary to the increasing concentration of the atoms on the left side of the plane, presuming that the gesture is unidimensional. Therefore, this jurisprudence states that atoms have a greater inclination to travel from higher to a lower part.
In order to show the diffusion procedure ( peculiarly, in gases ) , an experiment was conducted utilizing NH3( ammonium hydrated oxide ) and HCl ( hydrochloric acid ) in a glass tubing. A two pess glass tubing was fastened to a ring base. Each cotton balls were at the same time moistened with NH3and the other with HCl, both placed at the surrogate ends. In a twosome of proceedingss, a white fume all of a sudden formed inside the tubing as a consequence of the reaction between the NH3and HCl. The distance of the cotton ball moistened with HCl to the white fume was recorded every bit good as the several distance of NH3. Based on the recorded measurings, the white fume formed distantly to the cotton ball moistened with NH3( MW= 17 g/mole ) , which covered an mean distance of 20.85 centimeters compared to the mean distance of HCl, which was 14.45 centimeter. From the result of the experiment, it can be deduced that ammonium hydrated oxide diffused faster than hydrochloric acid. Therefore, a hypothesis was derived, “The molecular weight straight affects the rate of diffusion: the higher the molecular weight, the slower the diffusion and frailty versa.”
Sing that the speed of the Brownian motion of the atom is greater so the speed of diffusion itself, mensurating diffusion is by experimentation hard ( Jirgensons, 1954 ) . For the ground that diffusion rate is bantam, geting the certain or most likely rate of diffusion is non that easy. It is extremely needed to take the appropriate medium that will assist bring forth a conclusive and most logical consequence. Consequently, it was Graham, who pioneered the proposal of diffusion rate observation in gels alternatively of utilizing liquids as media. Gels are jointly known as colloids in a solid stage holding a retentive and stiff system with a scattering medium every bit good as a disperse part. Harmonizing to Hartman ( 1947 ) , proving diffusion utilizing gel as a medium is less hard than proving diffusion through a liquid. For the ground that convection currents and mechanical perturbations and quivers might impact the distribution of substances undergoing diffusion, if the procedure is tested through a pure liquid.
In add-on, the belongings of substance which involves its ability to scatter itself throughout the full part until its concentration reaches the point of equilibrium is called diffusivity of the substance. All atoms exhibit this same sort of belongings. However, the discrepancy is in the speed and the diffusion rate.
Diffusion can someway be related to some parametric quantities involved in viscousness, osmotic force per unit area, dialysis and ultrafiltration. Some factors considered in the aforesaid procedures can besides considered in finding diffusion rate. Hence, by sing these, there might be a great experimental easiness when obtaining the coveted measuring that will uncover conclusive consequences.
This survey aimed to find if molecular weight straight affects the diffusion rate of a substance. The aims were the followers:
- To stress the relationship of molecular weight to the rate of diffusion in a certain clip.
- To explicate whether the molecular weight has a monolithic influence in footings of diffusivity slowing.
MATERIALS AND METHODS
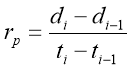 To find the consequence of molecular weight to the rate of diffusion of a certain substance, an agarose-water gel ( incorporating 99.8 % H2O and 0.2 % solid polyose ) was prepared which served as the the medium to prove diffusion rate. The medium was contained in a petri dish. Small sums of K permanganate, K bichromate and methylene were dropped at the same time on their several Wellss. The diameter was ab initio measured at 0 minute. Every after 3 proceedingss, the diameters of each dye were measured. The procedure was continuously repeated for 30 proceedingss. The partial and mean rate of diffusion were computed and tabulated. The undermentioned expression was used in calculating for the partial rate of diffusion:
To find the consequence of molecular weight to the rate of diffusion of a certain substance, an agarose-water gel ( incorporating 99.8 % H2O and 0.2 % solid polyose ) was prepared which served as the the medium to prove diffusion rate. The medium was contained in a petri dish. Small sums of K permanganate, K bichromate and methylene were dropped at the same time on their several Wellss. The diameter was ab initio measured at 0 minute. Every after 3 proceedingss, the diameters of each dye were measured. The procedure was continuously repeated for 30 proceedingss. The partial and mean rate of diffusion were computed and tabulated. The undermentioned expression was used in calculating for the partial rate of diffusion:
wherein: vitamin DI= diameter of coloured part at a certain clip
vitamin Di-1= diameter of coloured part instantly before vitamin DI
TI= clip when di was measured
Ti-1= clip instantly before TI
Graphs demoing the relationship of molecular weight to the mean rate of diffusion and the partial rate of diffusion within a certain clip were plotted and analyzed.
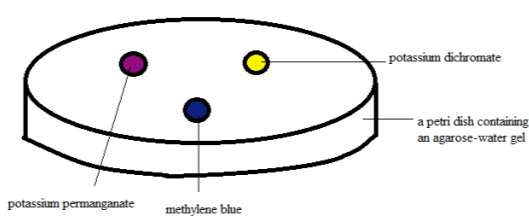 Figure 1. Diffusion test set-up at zero minute
Figure 1. Diffusion test set-up at zero minute
Figure 2. Diffusion test set-up after 30 proceedingss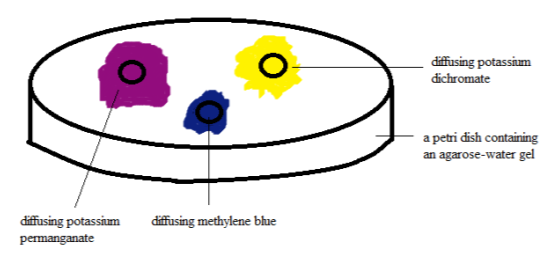
RESULTS AND DISCUSSION
Based on the consequences tabulated on Table 1.1, there was a sudden increase in the diameter of K permanganate, K bichromate and a little one in methylene blue. There were certain proceedingss in which the measured values of the diameter are unvarying and exhibited no alteration at all. The consequences besides showed that the scope of the diameter of each spreading substances varied with each other. The diameter of K permanganate ranged from 5-15 millimeter, K bichromate ranged from 5-14 millimeter while methylene blue merely ranged from 5-10 millimeter.
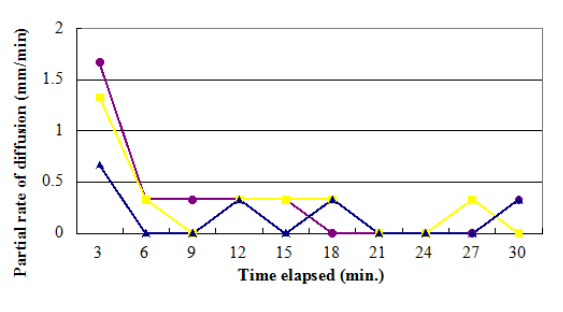
The values of the partial rate ( as shown on table 2 ) nevertheless, constituted a great discrepancy and some uniformities. On the contrary, the consequences clearly showed no tendency in malice of the increasing clip. The mean diffusion rate was computed from the obtained partial rates. Potassium permanganate had a value of 0.33 mm/min, K bichromate had a value of 0.30 mm/min, while methylene blue had a value of 0.17 mm/min.
The consequences hence showed how the diffusion speed and rate of a substance is being influenced by its molecular weight. It can be deduced that methylene blue ( methylthionine chloride ) had the lowest mean rate of diffusion knowing that its molecular weight is 374 g/mole and from this, it can besides be deduced that this substance diffused easy. Unwittingly, its molecular expression is C16Hydrogen18Nitrogen3SCl. Aside from the elements present in the substance, the molecular expression besides implies how big the atom is and how monolithic it is.
Consequently, the diffusion rate additions with diminishing molecular size ( Hauser, 1939 ) . And which in bend, molecular weight is extremely dependent on the
Table 1. Measured diameter of the diffusing substances in a certain clip
|
Time |
Diameter ( millimeter ) |
||
|
Potassium permanganate |
Potassium bichromate |
Methylene blue |
|
|
0 |
5 |
5 |
5 |
|
3 |
10 |
9 |
7 |
|
6 |
11 |
10 |
7 |
|
9 |
12 |
10 |
7 |
|
12 |
13 |
11 |
8 |
|
15 |
14 |
12 |
8 |
|
18 |
14 |
13 |
8 |
|
21 |
14 |
13 |
9 |
|
24 |
14 |
13 |
9 |
|
27 |
14 |
14 |
9 |
|
30 |
15 |
14 |
10 |
Table 2. Partial diffusion rate of each substance in a certain clip elapsed
|
Partial rates of diffusion ( mm/min. ) |
|||
|
Time elapsed ( min. ) |
Potassium permanganate ( MW= 158 g/mole ) |
Potassium bichromate ( MW= 294 g/mole ) |
Methylene blue ( MW= 374 g/mole ) |
|
3 |
1.67 |
1.33 |
0.67 |
|
6 |
0.33 |
0.33 |
0 |
|
9 |
0.33 |
0 |
0 |
|
12 |
0.33 |
0.33 |
0.33 |
|
15 |
0.33 |
0.33 |
0 |
|
18 |
0 |
0.33 |
0.33 |
|
21 |
0 |
0 |
0 |
|
24 |
0 |
0 |
0 |
|
27 |
0 |
0.33 |
0 |
|
30 |
0.33 |
0 |
0.33 |
|
Average rate of diffusion ( mm/min. ) |
0.33 |
0.30 |
0.17 |
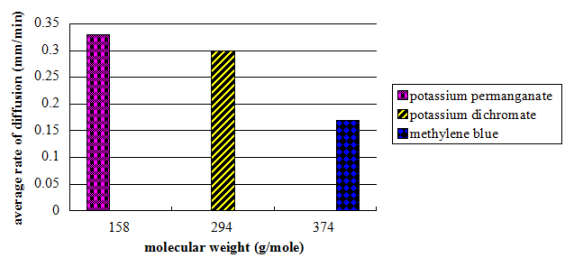
Figure 3. A saloon graph picturing the relationship of the molecular weight to the computed mean diffusion rate

Figure 4. A line graph demoing the partial rates of diffusion given a certain clip elapsed
molecular size. The greater the size of a molecule, the more monolithic it gets. However, it must be besides considered that gravitative acceleration has no direct influence in the molecular weight of a substance diffusing in a unidimensional way. Molecular weight is non dependent of gravitation though the term “weight” is being used. In chemical science, mass and weight are interchangeable. Therefore, the motion of atoms lessenings as the square root of the molecular weight is obtained ( Hauser, 1939 ) .
SUMMARY AND CONCLUSION
The consequence of molecular weight to the diffusion rate of a certain substance was once and for all ascertained. An agarose-water gel contained in a petri dish was used as a medium holed with three Wellss. Three assorted substances viz. , K permanganate, K bichromate and methylene blue were at the same time dropped on each several Wellss. The diameter of the diffusing substances were measured every after 180 seconds in 30 proceedingss. The partial rate of diffusion every bit good as the mean rate were computed.
Consequences showed that K permanganate, holding a molecular weight of 158 g/mole had the highest diffusion rate with the value of 0.33 mm/min. while the methylene blue, holding a molecular weight of 374 g/mole had the lowest diffusion rate with a value of 0.17 mm/min.
Therefore, the diffusion rate of a substance is clearly affected by its molecular weight. Nonetheless, molecular weight is non the lone factor that should be considered in finding the rate of diffusion. Several theoretical decisions were postulated sing the consequence of clip, temperature, concentration and etc. However, molecular weight besides contributes a big influence to the procedure. The finding of the consequence of molecular weight to diffusion might be utile in finding how certain belongingss affect the stimulation of other chemical procedures.
LITERATURE CITED
Ball, D. 2003. Physical Chemistry. Pacific, Grove, CA: Thomson Brooks/Cole. p. 674
Campbell, F. 2008. Elementss of Metallurgy and Engineering Alloys. USA: ASM
International. p. 66
Dean, R. 1948. Modern Colloids ( An Introduction to the Physical Chemistry of Large Molecules and Small Particles ) .USA: D. Van Nostrand Company, Inc. p. 35-37
Glasstone, S. , K.J. Laidler and H. Eyring. 1941. The Theory of Rate Processes ( The Kinetics of Chemical Reactions, Viscosity, Diffusion and Electrochemical Phenomena ) . USA: McGraw-Hill Book Company Inc. p. 516-517
Hartman, R. 1947. Colloid Chemistry. 2neodymiumedition. Boston: Houghton Mifflin Co. p. 434
Hauser, E. 1939. Colloidal Phenomena ( An Introduction to the Science of Colloids ) . USA: McGraw-Hill Book Company Inc. p. 78-79 ; 82
Jirgensons, B. and M.E. Straumanis. 1954. A Short Textbook of Colloid Chemistry. London: Pergamon Press LTD. p. 24 ; 38-39





