Each water molecule is joined to _____ other water molecules by ____ bonds.
“The Polarity of Water” a water molecule is joined to four other water molecules by hydrogen bonds.
b.two … hydrogen
—c.four … hydrogen
d.four … polar covalent
e.three … ionic
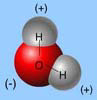
The unequal sharing of electrons within a water molecule makes the water molecule _____.
The electrons spend more time with the oxygen of the water molecule than with the hydrogens of water. Thus, the oxygen has a net negative charge and the hydrogens have a net positive charge.
b.change easily from a liquid to gaseous form
c.ionic
d.polar—
e.hydrophobic
b.polarity
c.tonicity
d.electronegativity—
e.ionic potential
b.hydrophilic
c.nonpolar covalent
d.hydrophobic
e.ionic

b.The insect is flying just above the water c.surface.
d.Surface tension.—
e.The insect is very light.
f.It is difficult to change the temperature of water.
b.Water molecules cling to plant cell walls
b. A drop of water spilled on a table forms a drop on the table, rather than spreading out over the surface
b. A sewing needle floats when it is placed gently on top of water in a bucket
(b) one mole of solute per liter of water.
(c) a one-to-one ratio of solute to solvent molecules.
Both (b) and (c).
None of the above.
b.Polar groups attract one another.—
c.Polar groups repel water.
d.Nonpolar groups attract one another.
e.All of the above.
b.a separation of molecules into neutral atoms.
c.breaking covalent bonds.
d.a change from a solid to a liquid.
e.a mingling of molecules and/or ions. —
Water is a source of ______________ for chemical reactions in cells.Many reactions incorporate O and H from water into biological molecules. This happens when you digest starch and protein, for example.
(b) oxygen atoms
(c) energy
Both (a) and (b)—
(a), (b), and (c).
Which statement is true of water’s tensile strength?Because of hydrogen bonding, water coheres to itself and adheres to cell walls. That makes it possible to pull water through plants without breaking the water column.
(b) It helps to pull water through plants.
(c) It involves both cohesion and adhesion.
Both (a) and (b).
(a), (b), and (c). —
Water has surface tension because …
The hydrogen bonds between surface water molecules are normally slightly stretched. Like a stretched sheet of rubber, the surface tends to contract and resists being penetrated.
b.hydrogen bonds between surface water molecules resist being stretched.
c.cohesion forces are weaker at the surface.
d.there is positive pressure inside the water mass.
e.molecules at the surface make more hydrogen bonds.
Which of the following helps most to explain why water has a high specific heat?When you heat water, much of the heat is used to break hydrogen bonds. Only the remaining heat can increase molecular motion, raising the temperature.
(b) The water molecule has exceptionally strong covalent bonds.
(c) Water temperature is exceptionally sensitive to heat.
Both (a) and (b).
Both (b) and (c).
Which factor is important in making it possible to cool yourself by sweating? Think carefully!
Random collisions allow some molecules to accumulate more energy than other molecules. The weakness of hydrogen bonds lets those molecules escape, leaving the cooler molecules behind.
(b) Hydrogen bonds are relatively weak.
(c) Water has more energy at the body surface.
Both (a) and (b).
(a), (b), and (c).—
Though you add heat, the temperature of boiling water remains constant because …
At boiling, all the added heat is used to break hydrogen bonds. Free of the water mass, the departing steam carries away all the added energy, with none left over to raise the temperature.
b.Water has a constant boiling temperature.
c.It takes energy to break covalent bonds.
d.It takes energy to break hydrogen bonds.—
e.None of the above. The temperature rises during boiling.
Which statement helps to explain why ice is less dense than liquid water?
The ice lattice has open spaces because of the angles at which hydrogen bonds form. Heat energy can break water molecules free of the lattice so they move into the openings.
(b) Cold molecules move less than warm molecules.
(c) Hydrogen bonds lengthen in the cold.
All of the above.
Both (a) and (b). —
The open spaces in water’s crystal structure make it possible for …
(b) water to have a low boiling point.
(c) life to occur in hot springs.
Both (b) and (c).
(a), (b), and (c).
Why doesn’t oil mix with water?
Water molecules cling to one another and won’t part to make room for uncharged (nonpolar) molecules. There’s no repulsion.
(b) Polar molecules repel nonpolar molecules.
(c) Polar molecules attract one another.—
(d) Nonpolar molecules attract one another.
Both (a) and (d).
Which property of water allows dogs to cool themselves by panting?Evaporative cooling takes place when molecules with the greatest kinetic energy leave a fluid as gas molecules. Because of water’s high heat of vaporization, a large amount of heat must be absorbed for 1 gram of water to be converted from liquid to gas. The dog loses this heat as the water on its tongue evaporates, allowing him to cool down by panting. To understand why this answer is correct, read about evaporative cooling.
b.the formation of covalent bonds between water molecules
c.water’s high surface tension
Which property of water allows a paper towel to pick up a puddle of water?
Water adheres to the cellulose molecules in a paper towel, allowing the towel to pick up a puddle of water. This is similar to the adhesion between water molecules and cellulose in cell walls responsible for water transport in plants.
b.water’s high heat of vaporization
c.adhesion of water molecules to other kinds of molecules—





