Introduction
Cardiovascular disease is the leading cause of death in both the UK and worldwide. The estimated cost is set to rise from $656 billion to $1208 billion in the following 15 years, putting the global healthcare system under huge pressure (Go et al., 2014).
Stem cells (SCs) have emerged as a possible treatment method and have been in clinical use for over a decade in cardiac intervention. Many variations between cell types, delivery methods, timing, number of cells, homing, and grafting highlight that stem cell therapy is plagued with unsolved issues (Shim et al., 2013). Whether the SC intervention is for acute myocardial infarction (AMI) or for coronary heart failure (CHF), its main therapeutic purpose is to repopulate the loss of 1 billion cardiomyocytes and supporting cells.
Cell Types Used
Adult SCs used can be categorized into bone marrow (BM)-derived, circulating, and resident to the heart. BM includes bone marrow mononuclear SCs (BMMNCs) and mesenchymal SCs (MSCs). Circulating SCs are represented by endothelial progenitor cells (EPCs), which have been shown to restore blood flow to necrotic myocardium, while cardiac stem cells (CSCs) purified from myocardium are believed to maintain cardiac regeneration abilities (Mirotsou et al., 2011).
Delivery Methods
Three distinct delivery methods have been established (fig. 1). The non-invasive and straightforward path is intracoronary injection but has issues including microvasculature occlusion. Direct or catheter-based intramyocardial injection allows inserted cells to enter the infarcted area, increasing grafting and homing of cells to the myocardium. Administration via catheter is the most common delivery method among trials due to its cost-effectiveness and repeatability.
Furthermore, direct intramyocardial injection during surgery has ethical issues relating to running a control group with fake surgery but has been shown to result in greater engraftment, especially for larger cells such as MSCs (Chong and Murry, 2014).
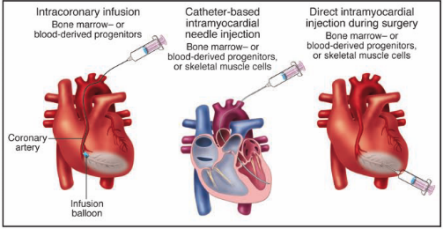
Figure 1 shows the current cell delivery routes: 1) Intracoronary extraction using an over-the-wire balloon catheter; 2) Intramuscular injection via catheter; or 3) Direct injection into the myocardium during surgery (Dimmeler et al., 2005).
First Generation Tests
First-generation bone marrow mononuclear cell (BMMNC)-based tests for acute myocardial infarction (AMI) with intracoronary injection had the common purpose of establishing if BMMNCs were capable of improving cardiac function.
Varying results highlight the difficulty in drawing conclusions from the data. A collection of tests (Strauer et al., TOPCARE AMI, BOOST & Fernandez et al.) reported practically identical results; 7-9% improvement in global left ventricular (LV) ejection fraction, significantly reduced end-systolic LV volumes, and improved perfusion in the infarcted area 4-6 months after transplantation (Shim et al., 2013).
Meanwhile, similar tests (TIME, Late TIME, HEBE, ASTMAI & Leuven AMI) reported no significant change in cardiac function (Behfar et al., 2014). Proposed reasons for result variations include cell timing, administration, and preparation. Furthermore, meta-analysis has highlighted a correlation between the number of disagreements within a test and the improvement in LV function (P = 0.005).
Additionally, the group of studies with no disagreements reported no improvement in LV function (Nowbar et al., 2014). This raises the question, do BMMNCs truly improve LV function, or is there another reason?
Paracrine Effect
The initial target of stem cell (SC) therapy was the physical replacement of damaged cells with transplanted ones. Now, research focus has moved towards harnessing the endogenous repair mechanisms of the transplanted cells through a paracrine effect (Menasche, 2014). This was believed to be the case because the number of SCs that regenerate in the myocardium following transplantation cannot account for the level of improvement (Mirotsou et al., 2011).
Rat models using mesenchymal stem cells (MSCs) have shown that soluble factors improve cardiac function. In particular, Fidelis-de-Oliveira et al. illustrated that isolating MSCs cultured medium (CM) following hypoxic conditions (hypoxia induces increased release of growth/angiogenic factors, cytokines, and vascular endothelial growth factor) promoted significant decreases in left ventricular end-diastolic pressure (35%), cardiac contractility and relaxation improvements (15% and 12% respectively) compared to CM under normoxia conditions (Fidelis-de-Oliveira et al., 2012). The results show that soluble factors released by MSCs are able to improve cardiac function following AMI.
Doyle et al. showed that conditioned media from endothelial progenitor cells (EPCs) could improve cardiac function in a porcine model following AMI (Figure 2). The exact mechanisms are unknown, but soluble factors such as vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF1), and transforming growth factor beta 1 (TGFβ1) secreted by EPCs with cardiotrophic and neoangiogenic effects are believed to be responsible for the infarct-related remodeling following MI (Doyle et al., 2008).
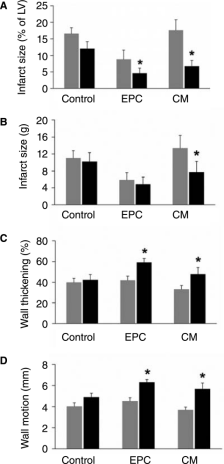
Figure 2 – Changes in infarct-related parts following AMI from baseline to 2 months post-therapy. Comparison across the scope of construction and maps known to be damaged following MI and comparing the intervention with endothelial progenitor cells (EPCs), conditioned media (CM) derived from EPCs, and standard control. Grey bars represent pre-treatment, and black bars indicate post-treatment. *p < 0.05 for comparing baseline to 2 months post-therapy.
Following coevals tests,
A distinguishable alteration in the biological agents used from non-cardiac (BMMNCs/MSCs/EPCs) to cardiac committed cells (CSCs, e.g., cardiospheres) has occurred, starting with the first-in-human test of CSCs harvested and transplanted during coronary artery bypass surgery (CABG) – the SCIPIO Trial. Interim results showed that LVEF and EF in the CSC-infused part of the infarct myocardium improved for CSC-treated groups.
Cardiac magnetic resonance (CMR) showed a significant increase in LVEF from 27.5±1.6% [Phosphorus=0.004, n=8] at 4 months to 35.1±2.4% [P=0.013, n=5] at 12 months after CSC extraction (Figure 4). Although patient numbers are low, these findings represent a significant discovery in the field and demonstrate a pronounced improvement in global and regional LV function, especially over time, indicating cardiac regeneration is possible (Chugh et al., 2012).
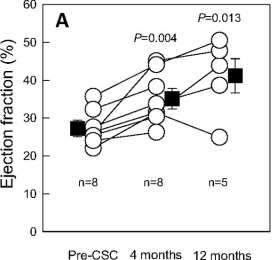
Figure 4 – Data from the SCIPIO Trial. Left Ventricular Ejection Fraction (LVEF) at baseline (27.5±1.6%), 4 months after cardiac stem cell (CSC) extraction (35.1±2.4%), and 12 months after CSC extraction (41.2±4.5%) (Chugh et al., 2012).
Controversy Around the SCIPIO Trial
In April of this year, the editors of the original paper (The Lancet) issued an expression of concern regarding the integrity of two auxiliary figures published online, which were carried out at a separate establishment from the main trial. Although they do not directly impact the findings, the controversy around them has cast doubt over the entire trial. A large number of clinical trials have been designed based on the SCIPIO trial’s results (cited 270 times), with a similar trial (CADUCEUS) unable to reproduce their findings of improved LV function following CSC graft, though they did find a decrease in scar tissue following MI (Makkar et al., 2012).
The inability to reproduce results and recent expression of concern highlight the difficulties following-generation cell therapy trials face. Further trials in different labs with higher patient numbers are required to determine the reliability and mechanism of action of CSCs in cardiac disease therapy.
Future Probes
Understanding the exact mechanisms of how soluble factors from each stem cell type improve cardiac map is needed. This could lead to replacing cell-based therapy with soluble factor therapy through the usage of biomimetic polymers to leverage the regenerative abilities of the soluble factors (Menasche, 2014).
Continuing to follow coeval tests to find implicit mechanisms of CSCs’ function in improving cardiac map and carrying on further tests with additional patients to increase the unity of data.
Introducing biomaterials as tools to increase cell retention and mediate soluble factor-controlled release once transplanted to optimize stem cell regeneration capabilities.
Conclusion
The initial belief that the mechanism of action of grafted stem cells generated neomyocardium has been altered to one in which an endogenous repair mechanism based on paracrine factors derived from the stem cells brings about the cardiac improvements. The cell type used has changed from non-cardiac to cardiac-committed (CSCs), which contain specific soluble factors capable of cardiac regeneration. Optimizing stem cell delivery to ensure cell grafting long enough to exhibit their paracrine effect is still a significant barrier. As the understanding of cardiac regeneration increases, more specialization of stem cell therapy for specific cardiac diseases will occur.
References
- Behfar, A., Crespo-Diaz, R., Terzic, A., & Gersh, B. J. (2014). Cell therapy for cardiac repair–lessons from clinical trials. Nat Rev Cardiol, 11, 232-246.
- Chong, J. J. H., & Murry, C. E. (2014). Cardiac regeneration using pluripotent stem cells—Progression to large animal models. Stem Cell Research.
- Chugh, A. R., Beache, G. M., Loughran, J. H., Mewton, N., Elmore, J. B., Kajstura, J., Pappas, P., Tatooles, A., Stoddard, M. F., Lima, J. A., Slaughter, M. S., Anversa, P., & Bolli, R. (2012).
- Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation, 126, S54-S64.
- Dimmeler, S., Zeiher, A. M., & Schneider, M. D. (2005). Unchain my heart: the scientific foundations of cardiac repair. The Journal of clinical investigation, 115, 572-583.
- Doyle, B., Sorajja, P., Hynes, B., Kumar, A. H., Araoz, P. A., Stalboerger, P. G., Miller, D., Reed, C., Schmeckpeper, J., Wang, S., Liu, C., Terzic, A., Kruger, D., Riederer, S., & Caplice, N. M. (2008). Progenitor cell therapy in a porcine acute myocardial infarction model induces cardiac hypertrophy, mediated by paracrine secretion of cardiotrophic factors including TGFbeta1. Stem Cells Dev, 17, 941-951.
- FIDELIS-DE-OLIVEIRA, P., WERNECK-DE-CASTRO, J.P., PINHO-RIBEIRO, V., SHALOM, B.C., NASCIMENTO-SILVA, J.H., COSTA E SOUZA, R.H., CRUZ, I.S., RANGEL, R.R., GOLDENBERG, R.C. & CAMPOS-DE-CARVALHO, A.C. 2012. Soluble factors from multipotent mesenchymal stromal cells have antinecrotic effects on cardiomyocytes in vitro and improve cardiac function in infarcted rat hearts. Cell Transplant, 21, 1011-21.
- GO, A.S., MOZAFFARIAN, D., ROGER, V.L., BENJAMIN, E.J., BERRY, J.D., BLAHA, M.J., DAI, S., FORD, E.S., FOX, C.S., FRANCO, S., FULLERTON, H.J., GILLESPIE, C., HAILPERN, S.M., HEIT, J.A., HOWARD, V.J., HUFFMAN, M.D., JUDD, S.E., KISSELA, B.M., KITTNER, S.J., LACKLAND, D.T., LICHTMAN, J.H., LISABETH, L.D., MACKEY, R.H., MAGID, D.J., MARCUS, G.M., MARELLI, A., MATCHAR, D.B., MCGUIRE, D.K., MOHLER, E.R. 3RD, MOY, C.S., MUSSOLINO, M.E., NEUMAR, R.W., NICHOL, G., PANDEY, D.K., PAYNTER, N.P., REEVES, M.J., SORLIE, P.D., STEIN, J., TOWFIGHI, A., TURAN, T.N., VIRANI, S.S., WONG, N.D., WOO, D., TURNER, M.B., AMERICAN HEART ASSOCIATION STATISTICS, C. & STROKE STATISTICS, S. 2014. Heart disease and stroke statistics — 2014 update: a report from the American Heart Association. Circulation, 129, e28-e292.
- MAKKAR, R.R., SMITH, R.R., CHENG, K., MALLIARAS, K., THOMSON, L.E., BERMAN, D., CZER, L.S., MARBAN, L., MENDIZABAL, A., JOHNSTON, P.V., RUSSELL, S.D., SCHULERI, K.H., LARDO, A.C., GERSTENBLITH, G. & MARBAN, E. 2012. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomized phase 1 trial. Lancet, 379, 895-904.
- MENASCHE, P. 2014. How Close Are We to Using Stem Cells in Routine Cardiac Therapy? Canadian Journal of Cardiology, 30, 1265-1269.
- MIROTSOU, M., JAYAWARDENA, T.M., SCHMECKPEPER, J., GNECCHI, M. & DZAU, V.J. 2011. Paracrine mechanisms of stem cell reparative and regenerative actions in the heart. J Mol Cell Cardiol, 50, 280-9.
- NOWBAR, A. N., MIELEWCZIK, M., KARAVASSILIS, M., DEHBI, H. M., SHUN-SHIN, M. J., JONES, S., HOWARD, J. P., COLE, G. D. & FRANCIS, D. P. (2014). Discrepancies in autologous bone marrow stem cell tests and enhancement of ejection fraction (DAMASCENE): weighted regression and meta-analysis. BMJ, 348, g2688.
- SHIM, W., MEHTA, A., WONG, P., CHUA, T. & KOH, T. H. (2013). Critical pathway in cardiac stem cell therapy: an update on cell delivery. Cytotherapy, 15, 399-415.





